Reading an MCA report is simple with a little guidance. Aster Bio’s Microbial Community Analysis (MCA) test involves extracting genetic material from the MLSS and then using the 16S rRNA regions for microbial identification. Sequencing the 16S region gives us the ability to identify all organisms in a complex mixed sample. The sequence data is then matched to the MiDAS database which contains genetic sequence information from worldwide wastewater treatment plants. The resulting output of the test is a total microbial census that gives a snapshot of which organisms are present and in what relative quantity at the sample time. The sequences are reported down to genus level with populations expressed as % of total reads.
Functional Groups

Above is the Functional Group table summarizing the test data. The functional groups listed are those most important to wastewater treatment system operations.
- AOB – Ammonia Oxidizing Bacteria – convert ammonia nitrogen to nitrite. Nitrosomonas is the most well-known AOB.
- NOB – Nitrite Oxidizing Bacteria – oxidize nitrite to nitrate. Some members of the most common wastewater genus, Nitrospira, are capable of also oxidizing ammonia to nitrate – Complete Ammonia Oxidizing Bacteria (COMAMMOX).
- Nitrate Reducers – under low D.O. or anoxic conditions, nitrate reducers use NO3/NO2 for respiration. Target end product is N2
- Sulfur Oxidizers – convert reduced sulfides to elemental sulfur and sulfate.
- Sulfur Reducers – use SO4 as an electron acceptor under anoxic/anaerobic conditions producing H2
- PO4 Accumulators – PAO organisms are important for biological phosphate removal and store phosphate in their cells.
- Glycogen Accumulators – GAO organisms occupy same ecological niche as PAO but do not store phosphate in their cells. High GAO populations can create problems in BNR systems.
- Bulking – This covers the non-filamentous bulking or Zoogleal type organisms. These microbes can produce excess EPS that interferes with both MBR and secondary clarification.
- Foaming – organisms that produce stable biological foam on aeration basins, generally due to hydrophobic cell surfaces. This includes Nocardia forms and parvicella.
- Filaments – organisms responsible for both floc macrostructure and filamentous bulking. Molecular testing complements traditional microscopic identification methods.
- Methylotrophs – microbes that thrive on one carbon compounds such as methanol or methane. Grow on some industrial wastewater compounds such as methylamine and dimethyl amine
Top Reads Table
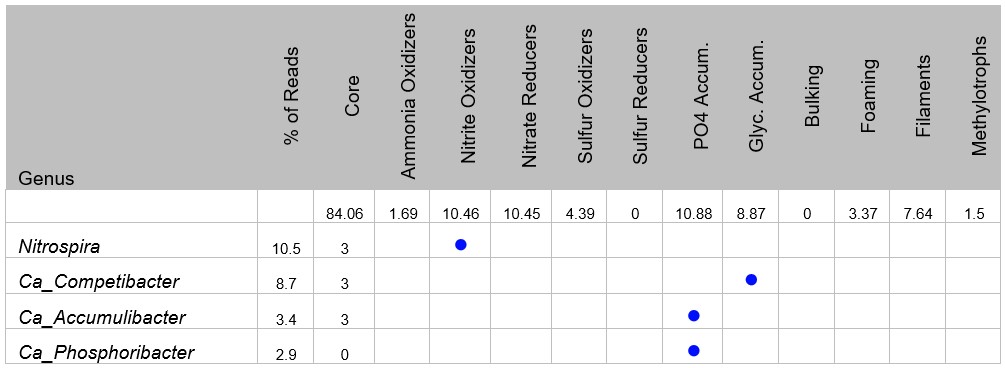
For a deeper dive into the MCA results, the next section contains the genera identified above 0.1% of total reads. Ranked according to commonness, additional information is provided on known metabolic properties. The known properties are based on MiDAS database information and peer-reviewed journal data.
Taxonomy Chart
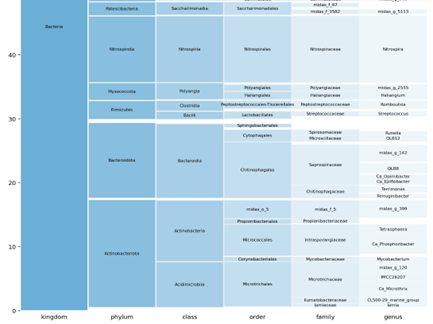
The Taxonomy Chart details the actual read from kingdom through more specific identification down to genus level. This allows us to see reads that may not be classified down to genus level while still allowing some inferences on potential metabolic roles. (The image below is cropped to fit)
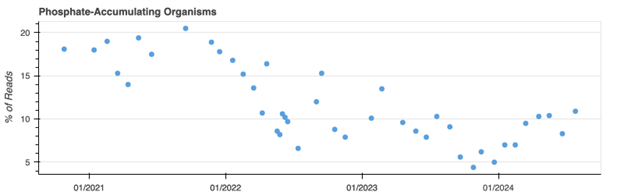
Aster Bio’s Online Tools for Time Series Data
The best way to use MCA is via time series data, where changes in functional groups over time can prove most useful. Using secure data base, Aster Bio’s customers can view graphs and look for specific trends. You can test drive a sample set online – Environmental Genomics MCA Sample Data Set
Putting MCA Results to Use
MCA testing gives vital data on the microorganisms growing in your wastewater system. By knowing the organisms present and how populations are moving over time, you can adjust inputs to optimize treatment efficiency. Examples of MCA results being used include:
- Adjusting MCRT to keep stable AOB & NOB populations in conditions where their growth rate slows. This avoids the potential for ammonia or nitrite breakthrough at the effluent.
- Monitoring if needed BNR populations of PAO vs GAO are being maintained with respect to sufficient organic acids and redox potentials in the anaerobic zone.
- Early warning for both non-filamentous and filamentous bulking potential based on genetic material in the MLSS.
- Evaluation of new waste streams and other operational changes on MLSS composition.

