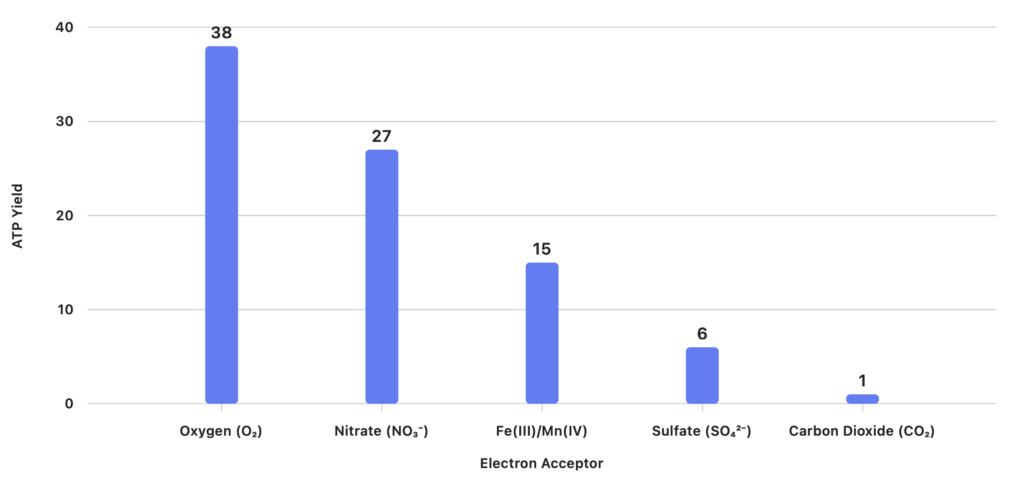
Energy Yield Comparison (ATP per Glucose) This bar chart compares the approximate ATP yield for each terminal electron acceptor used in wastewater treatment:
Biological wastewater treatment is fundamentally driven by microbial metabolism, where microorganisms transform pollutants into less harmful compounds. The efficiency and sustainability of these processes hinge on the selection and sequence of terminal electron acceptors (TEAs), which dictate both the energy yield for microbial growth and the nature of the metabolic end products.
The Electron Tower: Mapping Redox Potential
Central to understanding microbial energetics is the “electron tower,” a conceptual framework ranking redox couples by their standard reduction potentials (E°’). Electron donors, such as organic matter in wastewater, occupy higher positions (more negative potentials), while electron acceptors are found lower on the tower (more positive potentials). The energy released during electron transfer is proportional to the difference in potential (ΔE), as described by ΔG = -nFΔE, where n is the number of electrons and F is Faraday’s constant.
In practical terms, electrons “fall” from high-energy donors to low-energy acceptors, analogous to water flowing downhill. The choice of acceptor not only determines the energy yield but also influences the treatment process’s efficiency and the final metabolic products.
Hierarchy of Terminal Electron Acceptors and Energy Yields
Microbial communities in wastewater systems utilize electron acceptors in a preferential sequence, optimizing energy extraction and shaping treatment outcomes such as nutrient removal and odor control. The following hierarchy summarizes key TEAs, ordered by descending energy yield:
1. Oxygen (O₂)
- Redox Potential: +0.82 V (O₂/H₂O)
- Energy Yield: Highest; ~38 ATP per glucose
- Role: Enables complete oxidation of organics to CO₂ and H₂O. Aerobic processes (e.g., activated sludge) are highly effective but energy-intensive due to aeration requirements.
2. Nitrate (NO₃⁻)
- Redox Potential: +0.74 V (NO₃⁻/N₂)
- Energy Yield: Moderate; ~26–28 ATP per glucose
- Role: Used by denitrifying bacteria in anoxic conditions, crucial for nitrogen removal and prevention of eutrophication. Preferred after oxygen is depleted.
3. Manganese (Mn(IV)) and Iron (Fe(III))
- Redox Potentials: +0.41 V (MnO₂/Mn²⁺), +0.77 V (Fe³⁺/Fe²⁺)
- Energy Yield: Moderate
- Role: Important in anaerobic sediments and groundwater remediation, less common in mainstream wastewater due to limited availability.
4. Sulfate (SO₄²⁻)
- Redox Potential: -0.22 V (SO₄²⁻/HS⁻)
- Energy Yield: Low; ~5–6 ATP per glucose
- Role: Sulfate-reducing bacteria produce hydrogen sulfide, leading to odor and corrosion issues. Used in anaerobic digesters for sludge stabilization.
5. Carbon Dioxide (CO₂)
- Redox Potential: -0.43 V (CO₂/CH₄)
- Energy Yield: Lowest; ~1 ATP per acetate molecule
- Role: Methanogens generate methane, valuable for energy recovery in anaerobic digestion. Methane can be captured for electricity generation, supporting energy-positive treatment plants.
This sequential utilization—termed terminal electron-accepting processes (TEAPs)—ensures optimal energy extraction as conditions shift from aerobic to anaerobic.
Engineering Implications: Why Energy Yield Matters
The energy yield from different TEAs is a critical parameter in designing efficient and sustainable wastewater treatment systems. High-yield acceptors like oxygen accelerate pollutant degradation but require significant energy input for aeration, often accounting for 50–60% of a plant’s electricity consumption. Conversely, lower-yield anaerobic processes reduce operational costs and enable resource recovery, such as biogas production.
Understanding the electron tower also aids in predicting microbial community dynamics. For example, in high-organic-load wastewaters, depletion of high-yield acceptors leads to methanogenesis, but imbalances can result in volatile fatty acid accumulation or sulfide toxicity. Engineers can manipulate system conditions—such as nitrate addition to inhibit sulfate reduction—to optimize energy yields and minimize operational challenges.
